SA Political Research - COVID-19 Vaccine Supply
This Political Research Note was prepared by JP Landman in his personal capacity. JP is an independent political and economic analyst and the opinions expressed in this article are his own and do not reflect the views of the Nedbank Group.
A proverbial storm has developed around South Africa’s procurement, or the perceived lack thereof, of Covid-19 vaccines. People are understandably anxious. I therefore compiled this note from publicly available information, and trust readers find it useful.
What is approved and available?
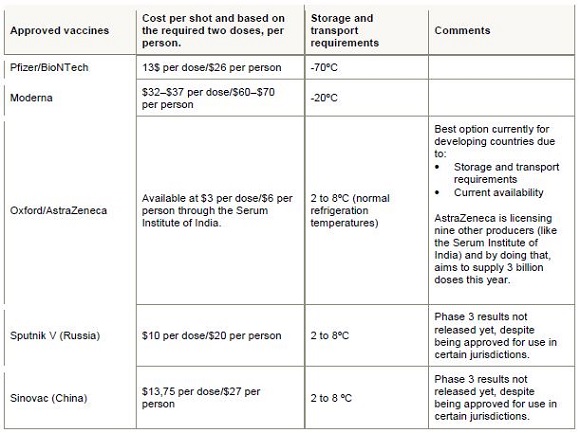
Currently (though this changes all the time), there are five approved vaccines available in the world. All of them require two shots, administered roughly three weeks apart. One must therefore distinguish between doses and number of people treated – two doses are needed for each person.
What is still in development?
The World Health Organisation (WHO) reports that some 50 vaccines are in development worldwide, so more and cheaper vaccines are sure to follow.
- The Johnson & Johnson vaccine will require only one shot, but it is still in its final trials and has not been approved yet. (Johnson & Johnson has submitted their vaccine for approval in South Africa and the Aspen factory in Nelson Mandela Bay is under licence to manufacture it. Dr Glenda Gray is running the clinical trials for Johnson & Johnson in South Africa.)
- Other Chinese companies apart from Sinovac are also developing vaccines, as are companies in India.
What has been/is being distributed?
All vaccines must be cleared for use by the regulatory authorities in the recipient country. While the numbers change daily, 33 countries have acquired one or more of the five approved vaccines to date and have started inoculating citizens. Thirty countries are using Pfizer/BioNTech, four are using Sputnik V, one Sinovac and two Moderna. (Some countries use more than one vaccine.)
In practice, some countries are doing much less than the headlines suggest. Hungary has less than 5 000 people on Pfizer/BioNTech and another 3 000 on Sputnik; Mexico has 1 500 people on Pfizer/BioNTech (paid for by a private philanthropist). Brazil has 6 million doses for 220 million people, but nothing has been distributed yet since the Brazilian regulatory authority has not approved it. The European Union acquired Pfizer/BioNTech vaccines centrally and started distribution among some of its members at the end of December 2020. (President Ramaphosa has launched a similar initiative for the African continent – see below.)
Global supply
Wealthier countries are obviously better placed to buy the vaccines. To date, 53% of the estimated supply of all vaccines in phase three clinical trials (not just the five approved to date), has already been bought by countries that make up only 14% of the world’s population. Some countries bought enough doses to vaccinate their population three times. Canada bought for five times its population, the US for about three times, and the UK for two times. At some point vaccines are going to be given away…
The springbok and wildebeest of the plains know that there is safety in numbers. The same goes for vaccines – pooling resources gives one a better chance of obtaining a vaccine. The best chance for poorer countries to acquire vaccines is through the Covax programme run by the WHO and GAVI, which involve 64 countries, philanthropic foundations and manufacturers. The richer countries pay more to subsidise poorer countries, and donors like the Bill & Melinda Gates Foundation also contribute. To date, 2 billion doses have been acquired for Covax (enough to cover one billion people). South Africa is participating in this programme and has paid the 15% deposit required (R283 million). The country will receive doses for 6 million people.
On 8 November 2020, before any vaccines were available, President Ramaphosa set up the African Union (AU) programme to acquire vaccines for the continent at large. It supplements Covax. To date, 270 million doses have been acquired from AstraZeneca (through the Serum Institute of India), Pfizer and Johnson & Johnson (although the latter is not approved yet). It will be distributed according to population size (not case load) and South Africa will receive enough doses to vaccinate a further 6 million people. Finance will be available through the African Export Import Bank, repayable over five years. The AU is also negotiating with the World Bank on behalf of African countries for access to a further $5 billion in financing for vaccines.
South Africa
Between these two collective schemes 12 million South Africans will be vaccinated.
The country is also negotiating with individual suppliers outside these initiatives. Vaccines for a further 750 000 people (1,5 million doses) will come from the Serum Institute of India – Treasury has confirmed that the terms of agreement have been agreed and delivery is expected in February. The table below summarises the status quo:

Clearly, the popular narrative that South Africa has missed the boat because we did not deal with individual suppliers, is a bit off the mark.
Procurement is also not straightforward. The Serum Institute wants 100% payment upfront (which is tricky within Treasury rules), while Johnson & Johnson (pending approval) requires an insurance/indemnity arrangement should there be negative consequences from the use of the vaccine. Prices also vary widely – in December it was leaked in Belgium that various vaccine prices varied from $2,15 to $21,78 per shot! Easy to pay more than you have to.
The 12,75 million people for whom vaccines have been procured is about one third of the target population of 40 million government wishes to vaccinate. So, vaccines for another 18 million people will still have to be procured through Covax, the AU and direct negotiations.
The target of 40 million may be too ambitious. There are only about 40 million adults in the country and the vaccines are not authorised for children under 16. That implies a vaccination rate of 100% for all adults. (Judging by the number of teenagers infected at rage parties one can only hope for a speedy under-16 approval.)
The country’s 1,5 million health workers will be first in line for vaccination, followed by 16,5 million essential workers, people with co-morbidities and people over the age of 60. We should know by April when all the over 60s will be vaccinated.
Current estimates are that the vaccination programme will cost a total of about R10 billion. The money will be provided for in the February budget together with whatever other financing mechanisms are being considered.
More than vaccines
With enough doses in the pipeline for 12,75 million South Africans, and more vaccines likely to be available soon, the attention must switch to the logistics of getting those doses from OR Tambo Airport to Kakamas and Mthatha and Ulundi. Not just the doses – also the syringes, the personnel and all other resources needed. It is a daunting logistical task – more so than the 2010 World Cup or the 1994 election, as the president pointed out. He therefore appointed an inter-ministerial cabinet committee to oversee the logistics. It also gives journalists a point of entry to keep the public informed.
Speed of development
A perspective on dates is in order. It took the world about 11 months to develop and clinically test the first vaccine. That is extraordinary. The vaccine for the 1918 flu epidemic was only made available in 1945. It is a singular achievement for mankind that this vaccine was developed in such a short time.
So what?
- South Africa’s strategy has been to procure vaccines through two collective schemes: Covax and the AU. This has secured vaccines for 12 million people.
- The next step is to procure directly from individual manufacturers as their products become available. The first direct agreement is with the Serum Institute of India, under licence from Oxford/AztraZeneca, and will deliver enough doses for 750 000 people (earmarked for health workers).
- Delivery will be between February and June, and by June we will have a good idea of how inoculation is proceeding.
- Supply of vaccines will increase globally and in South Africa. The Johnson & Johnson vaccine is in its final trials and up for approval. The Sputnik vaccine is being tested outside Russia, as is the Chinese vaccine (Sinovac). More vaccines are being developed by other players. It is now a scramble, but there will be an abundance of supply. (And that is before the countries that bought in excess start giving away vaccines.)
- Prices vary widely, and as supply increases prices should come down further.
- The remaining challenge for South Africa is to manage the coordination and distribution of vaccines. One step at a time.

Author: JP Landman - Political Analyst
This communication is issued by Nedgroup Private Wealth (Pty) Limited and its subsidiaries (Nedbank Private Wealth) for information purposes only, and recipients should not rely on the information as advice without obtaining financial, tax or other professional advice. The information has been obtained from various sources and may include facts and events or prevailing market conditions at the time or date of the information going to print. Nedbank Private Wealth does not warrant the completeness or accuracy of any of the information and does not make any representation that the information is appropriate for use by investors in all jurisdictions. All opinions expressed and recommendations made are subject to change without notice. Nedbank Private Wealth and its employees may hold securities or financial instruments mentioned herein. The information in this document does not constitute an offer or solicitation of financial services or products and Nedbank Private Wealth accepts no liability for any loss or damage, including loss of profits or any type of financial or other pecuniary or direct or special indirect or consequential loss arising, whether in negligence or for breach of contract or other duty as a result of use or reliance on the information in this communication. Nedbank Private Wealth, an authorised financial services provider through Nedgroup Private Wealth Pty Ltd Reg No 1997/009637/07 (FSP828), registered credit provider through Nedbank Ltd Reg No 1951/000009/06 (NCRCP16), and member of JSE Ltd through Nedgroup Private Wealth Stockbrokers Pty Ltd Reg No 1996/015589/07 (NCRCP59).






